How to find specific heat of a turpentine question?
A copper cylinder has a mass of 76.8 g and a specific heat of 0.092 cal/g ⁰C. It is heated to 86.5⁰ C and then put in 68.7 g of turpentine whose temperature is 19.5 ⁰ C. The final temperature of the mixture is 31.9⁰ C. What is the specific heat of the turpentine? Use the formula Q1=Q2 -> mATc =mATc. Provide correct units and show work.
 Jimmycallens
Jimmycallens
12
Answer
Answers can only be viewed under the following conditions:
- The questioner was satisfied with and accepted the answer, or
- The answer was evaluated as being 100% correct by the judge.
4.8K
The answer is accepted.
Join Matchmaticians Affiliate Marketing
Program to earn up to a 50% commission on every question that your affiliated users ask or answer.
- answered
- 3174 views
- $4.00
Related Questions
- Determine the angle
- Algebra Word Problem #1
- Solve $abc=2(a-2)(b-2)(c-2)$ where $a,b $ and $c$ are integers
- Transformations of Parent Functions
- When is Galois extension over intersection of subfields finite
- How to properly write rational exponents when expressed as roots?
- Length of a matrix module
- Length of a matrix module
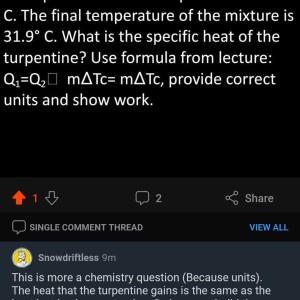

Could you comment the formula? There seems to be a missprint.
Q1=Q2 -> mATc= mATc. The A is like a triangle but I can't produce the triangle on my phone
You may also upload an screenshot or a picture.
Uploaded the pic